INTRODUCTION
Adolescents and young adult (AYA) survivors of Hodgkin lymphoma (HL) are potentially at increased risk of cardiovascular (CV) disease due to anthracycline exposure, in addition to use of mediastinal radiotherapy (RT). Although the risk has been well described in the pediatric age-group, the impact in the AYA population has been less well characterized. Capturing the incidence of these late effects is challenging given that events can occur more than a decade after therapy completion. Using population-based administrative data, we evaluated the incidence of CV disease (combined heart failure (HF) and ischemic heart disease (IHD)) in a cohort of AYA survivors treated for classical HL (cHL) using ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or equivalent chemotherapy.
METHODS
Patients with cHL aged 16-39 years (y), diagnosed between 1992-2013 and treated with an ABVD or equivalent therapy, were identified in the BC Cancer Lymphoid Cancer Database. Patients must have survived to an Index Date defined as 2 y from most recent HL event (primary diagnosis or if applicable, most recent relapse) and have had a minimum follow-up of 1 y beyond their Index Date. Patients were excluded if they had history of prior malignancy or HIV positivity. Limited stage disease was defined as stage IA, IB or IIA and absence of bulky disease (≥10cm); all others had advanced stage disease.
Cases were linked with population-based databases of BC Cancer Registry; BC Radiation Oncology Database; and BC Ministry of Health (MOH) Chronic Disease Registry (CDR) that captures all BC residents registered with medical service plan coverage during the study period. The outcome variables, including HF and IHD, were defined by the BC MOH CDR using Standardized Case Definitions. To focus on late onset CV complications, only events that occurred after the Index Date were included in the analysis. A 10:1 individually-matched control population was identified from the CDR based on age, sex, and health authority region on the Index Date of the matched case. Controls were excluded if they had a pre-existing malignancy, HF, or IHD prior to the study window. Individual outcomes were collected from the Index Date of the matched case until December 31, 2015 or until an individual was censored due to loss to follow-up or death. Kaplan Meier (K-M) methodology and log-rank test was used to estimate cumulative incidence. A competing risk regression analysis was used to evaluate relative risk (RR) and p-values less than 0.05 were considered significant.
RESULTS
With a median follow-up time of 11 y (range 3-24 y) from most recent HL event, 764 AYA 2-y survivors were identified, aged 20 to 61 y (median 38 y) at the end of study period. The proportion of limited and advanced stage disease was 34.2% and 65.6%, respectively; and 49.9% were male. Eighty-eight patients (11.5%) had relapsed disease; eighty-six (11.3%) underwent high dose chemotherapy and autologous stem cell transplantation as part of their salvage therapy. In total, 268 patients (36.4%) were treated with mediastinal RT for primary therapy or for relapsed disease. Fifty-three percent received cumulative anthracycline dose ≥300 mg/m2.
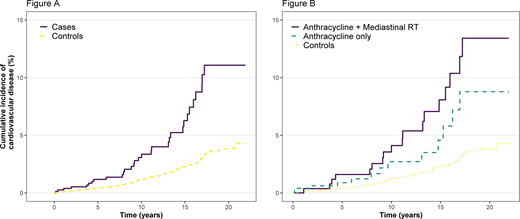
Survivors had a 3-fold increased risk of CV disease relative to controls (p<0.0001). The onset of CV disease in survivors occurred at median of 11.7 y after most recent treatment (range 2.2-19.2 y), and at a median age of 44.3 y (range 21 - 58 y). At 15 y, the estimated cumulative incidence of CV disease was 6.3% in survivors compared to 2.3% in controls (Figure A). In the 496 survivors that received chemotherapy only, the incidence of CV disease at 15 y was 4.6% vs 2.3% in controls, and those that received anthracyclines and mediastinal RT had significantly higher incidence at 8.6% (Figure B). The increase in risk was greatest for a diagnosis of HF (RR 6.92, p<0.0001): at 15 y, the cumulative incidence of HF was 2.2% vs 0.6% in controls. The RR of IHD was 2.63 (p<0.0001) with incidence of 5.1% in cases compared to 1.8% in controls.
CONCLUSION
Similar to the pediatric population, AYA cHL survivors are at increased risk of both HF and IHD after completion of treatment. The majority of patients had received ABVD alone and had a lower incidence of CV disease at 15 y when compared to those that received treatment that included mediastinal RT. These results will inform counseling regarding risk factor modification and aid in the development of surveillance guidelines for AYA survivors.
Gerrie:Sandoz: Consultancy; Roche: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Astrazeneca: Consultancy, Research Funding. Villa:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; AZ: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria; Nano String: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Sandoz Canada: Consultancy, Honoraria; Immunovaccine: Consultancy, Honoraria; Purdue Pharma: Consultancy, Honoraria. Scott:NIH: Consultancy, Other: Co-inventor on a patent related to the MCL35 assay filed at the National Institutes of Health, United States of America.; Roche/Genentech: Research Funding; Celgene: Consultancy; NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoString, Research Funding; Abbvie: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy, Research Funding. Sehn:AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Acerta: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Teva: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; Verastem Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria; Chugai: Consultancy, Honoraria. Savage:BeiGene: Other: Steering Committee; Roche (institutional): Research Funding; Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie, Servier: Consultancy; Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal